Introduction:
Organic chemistry is a vast and crucial branch of chemistry dealing with the structure, properties, and reactions of organic compounds, primarily those containing carbon and hydrogen atoms.
Within this domain, alkyl halides and aryl halides represent a significant class of organic molecules with unique characteristics and diverse applications.
This article delves into the key aspects of alkyl halides and aryl halides, exploring their fundamental properties, contrasting features, and reactivity patterns.
Alkyl Halides:
➡️ Definition: Alkyl halides, also known as haloalkanes, are organic compounds derived from alkanes (saturated hydrocarbons) by replacing one or more hydrogen atoms with halogen atoms (fluorine, chlorine, bromine, or iodine).
➡️ General Formula: R-X, where R represents an alkyl group and X represents a halogen atom.
➡️ Examples: Chloromethane (CH3Cl), bromoethane (CH3CH2Br), iodoform (CHI3).
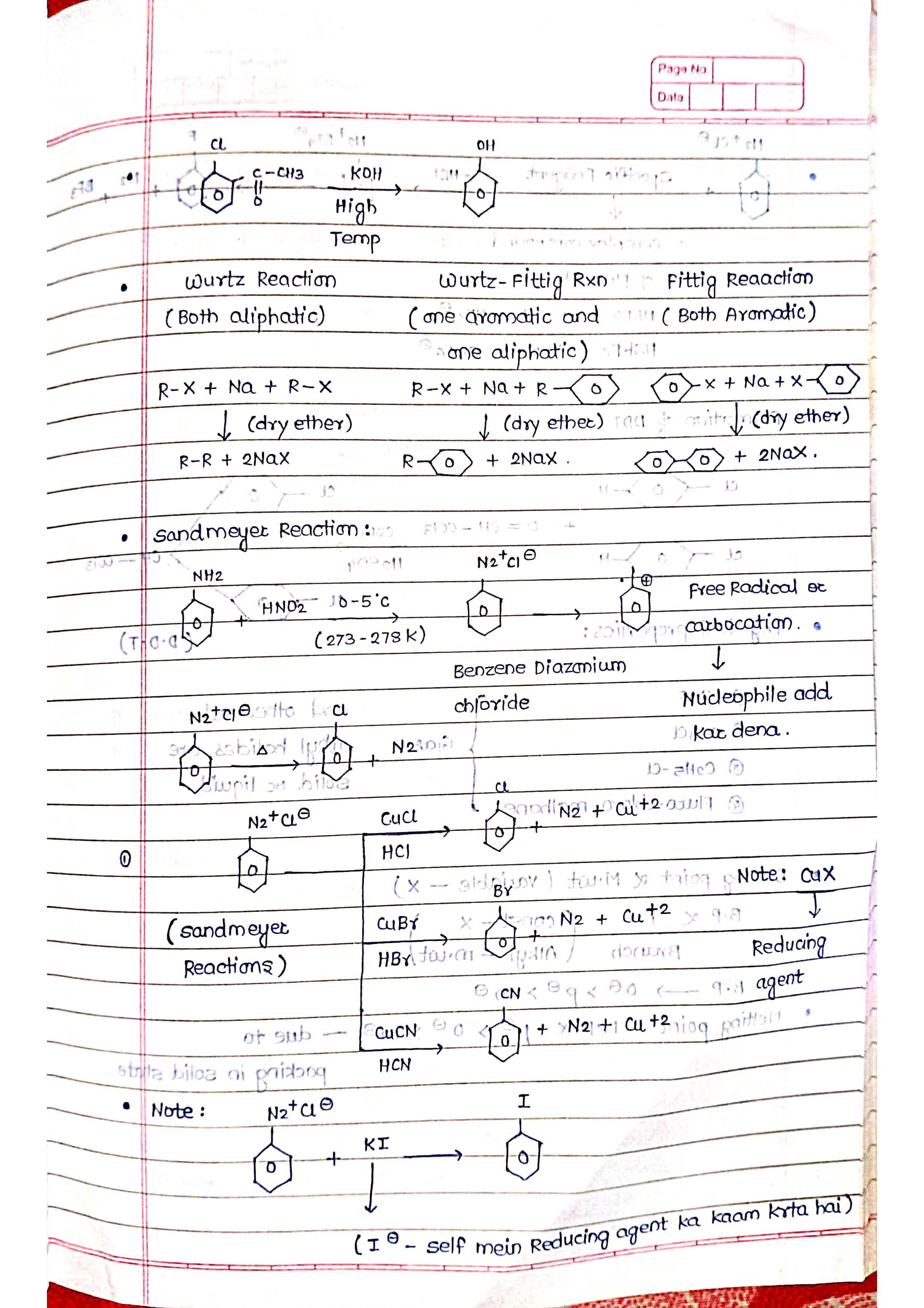
➡️ Preparation: Various methods exist for synthesizing alkyl halides, including halogenation of alkanes, reaction of alcohols with halogenating agents, and nucleophilic substitution reactions.
➡️ Physical Properties: Alkyl halides generally exhibit lower boiling points and higher melting points compared to their corresponding alkanes due to polar C-X bonds.
➡️ Chemical Properties: Alkyl halides are highly reactive due to the presence of the polar C-X bond, readily undergoing nucleophilic substitution reactions where the halogen atom is replaced by another nucleophile.
Aryl Halides:
➡️ Definition: Aryl halides, also known as haloarenes, are organic compounds derived from aromatic hydrocarbons (compounds containing benzene rings) by replacing one or more hydrogen atoms with halogen atoms.
➡️ Structure: Unlike alkyl halides, aryl halides lack a specific general formula due to the varying structures of aromatic rings.
➡️ Examples: Chlorobenzene (C6H5Cl), bromotoluene (C6H4CH3Br), iodophenol (C6H5IO).
➡️ Preparation: Similar to alkyl halides, aryl halides can be synthesized through various methods, including direct halogenation of aromatic rings, diazonium salt reactions, and Sandmeyer reaction.
➡️ Physical Properties: Aryl halides generally possess higher boiling points and melting points compared to alkyl halides due to the stronger C-X bond and the presence of aromatic rings.
➡️ Chemical Properties: Aryl halides exhibit similar reactivity patterns to alkyl halides, participating in nucleophilic substitution reactions. However, the presence of the aromatic ring can influence the reaction rates and mechanisms compared to their aliphatic counterparts.