Viscosity - Fluids, encompassing both liquids and gases, are a fundamental part of our world. Their behavior plays a crucial role in various scientific and engineering applications. One key property that defines how fluids behave is viscosity. Viscosity essentially captures a fluid's resistance to flow or deformation under stress. In simpler terms, it reflects how "thick" or "thin" a fluid is. This article delves into the world of viscosity, exploring its definition, different types, real-world examples, and its practical applications across various disciplines.
Definition of Viscosity
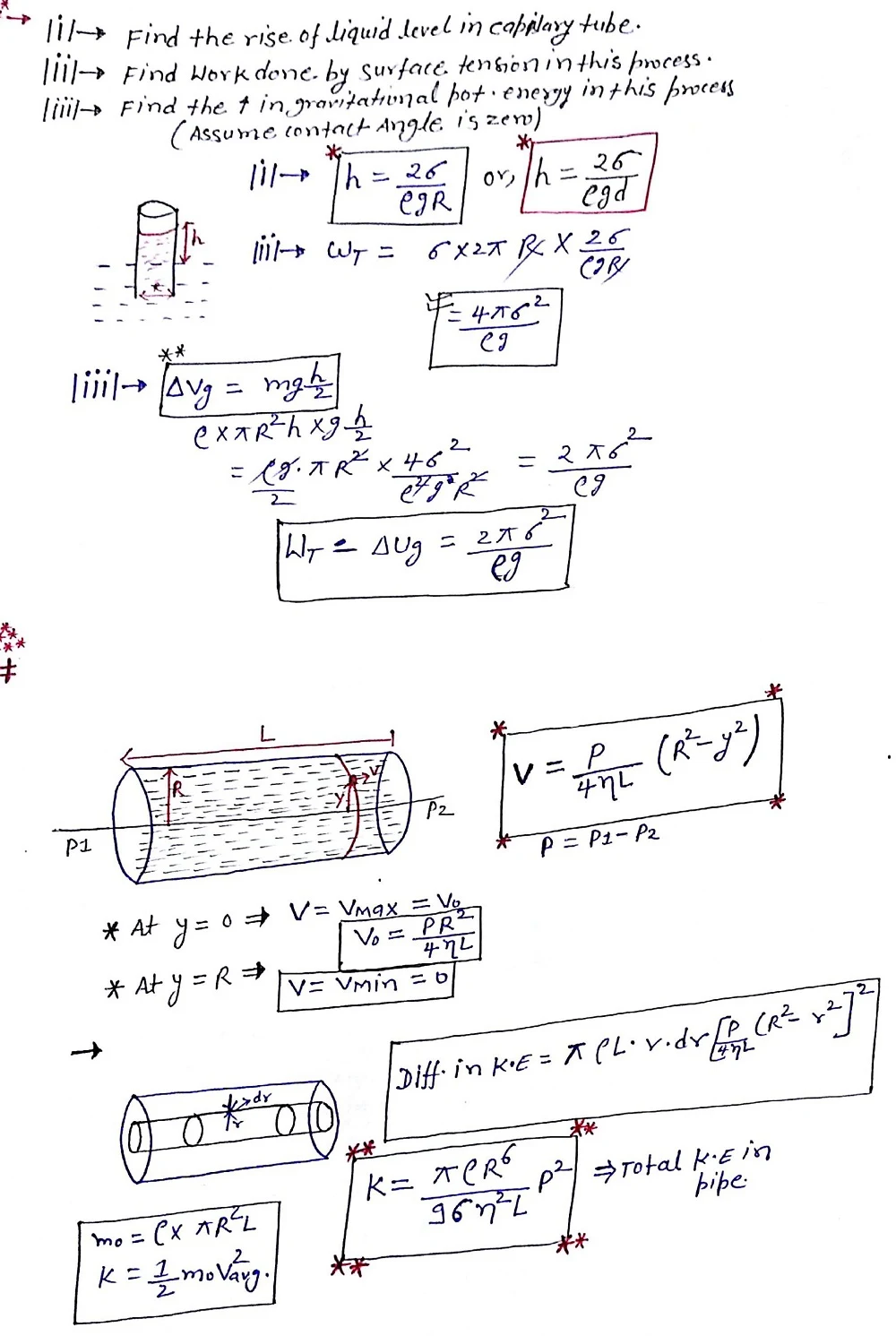
Scientifically, viscosity is defined as a measure of a fluid's resistance to deformation at a given rate. It quantifies the internal frictional force between adjacent layers of the fluid that are in relative motion. Imagine a liquid flowing through a pipe. The layers closer to the center experience less resistance and flow faster, while those near the walls encounter friction from the pipe surface and flow slower. Viscosity essentially reflects the ease or difficulty with which these layers slide past each other. Fluids with high viscosity resist this internal friction more, making them flow sluggishly (think honey). Conversely, fluids with low viscosity flow more readily (think water).
Types of Viscosity
There are two main types of viscosity to consider:
⦿ Dynamic Viscosity (absolute viscosity): This is the SI unit of viscosity, measured in Pascal-seconds (Pa·s). It represents the internal friction between fluid layers and is the most fundamental measure of viscosity.
⦿ Kinematic Viscosity: This is the ratio of dynamic viscosity to the fluid's density and is measured in square meters per second (m²/s). It reflects the fluid's flow characteristics independent of its density.
Examples of Viscosity in Everyday Life
Viscosity plays a role in numerous aspects of our daily lives. Here are a few examples:
⦿ Honey vs. Water: Honey has a high viscosity, making it slow to flow and cling to surfaces. Water, on the other hand, has a low viscosity and flows freely.
⦿ Motor Oil: Different grades of motor oil have varying viscosities. Thicker oils (higher viscosity) are better suited for cold starts, while thinner oils (lower viscosity) improve fuel efficiency.
⦿ Blood Flow: Blood viscosity is crucial for proper blood circulation. Abnormal viscosity levels can lead to health problems.
⦿ Painting Consistency: Paints with high viscosity leave thicker coats and require more effort to spread.
Applications of Viscosity
Understanding viscosity is essential in various fields:
⦿ Chemical Engineering: Viscosity is crucial in designing pipelines, optimizing flow rates, and formulating products with desired consistency.
⦿ Food Science: Viscosity plays a role in food texture, processing, and shelf life.
⦿ Lubrication: Lubricants with appropriate viscosity are essential for reducing friction and wear in machinery.
⦿ Cosmetics: Viscosity determines the feel and application of creams, lotions, and other personal care products.
Formula Table
Here's a table summarizing the formulas related to viscosity:
This table provides a quick reference for understanding the mathematical relationships between viscosity and other fluid properties.
By understanding viscosity, we gain valuable insights into the behavior of fluids and their applications in various contexts. From the flow of blood in our bodies to the smooth operation of machinery, viscosity plays a significant role in our world.
Viscosity FAQs
What is the relationship between viscosity and temperature?
In most fluids, viscosity decreases with increasing temperature. As a fluid heats up, the molecules move faster and experience less intermolecular attraction. This reduces the internal friction between layers, allowing them to flow more easily. Conversely, colder temperatures lead to higher viscosity as the molecules move slower and stick together more.
Can gases have viscosity?
Yes, gases also exhibit viscosity, although to a much lesser extent compared to liquids. In gases, the molecules are farther apart and interact less frequently, resulting in lower internal friction.
How is viscosity measured?
There are various instruments used to measure viscosity, such as viscometers and rheometers. These instruments measure the force required to move a fluid at a specific rate or the rate of deformation under a specific stress.
What are some factors that affect viscosity?
Several factors can influence a fluid's viscosity, including:
⦿ Temperature: As discussed earlier, temperature has a significant impact on viscosity.
⦿ Molecular size and shape: Larger and more complex molecules typically lead to higher viscosity.
⦿ Presence of additives: Adding thickeners or thinners to a fluid can significantly alter its viscosity.
⦿ Pressure: In some liquids, viscosity can increase slightly with increasing pressure.
What happens if a fluid has very high viscosity?
Fluids with extremely high viscosity can behave almost like solids. They may require significant force to flow and exhibit very slow movement.
What happens if a fluid has very low viscosity?
Fluids with very low viscosity are highly fluid and flow readily with minimal resistance. They may struggle to provide lubrication or maintain a desired shape.