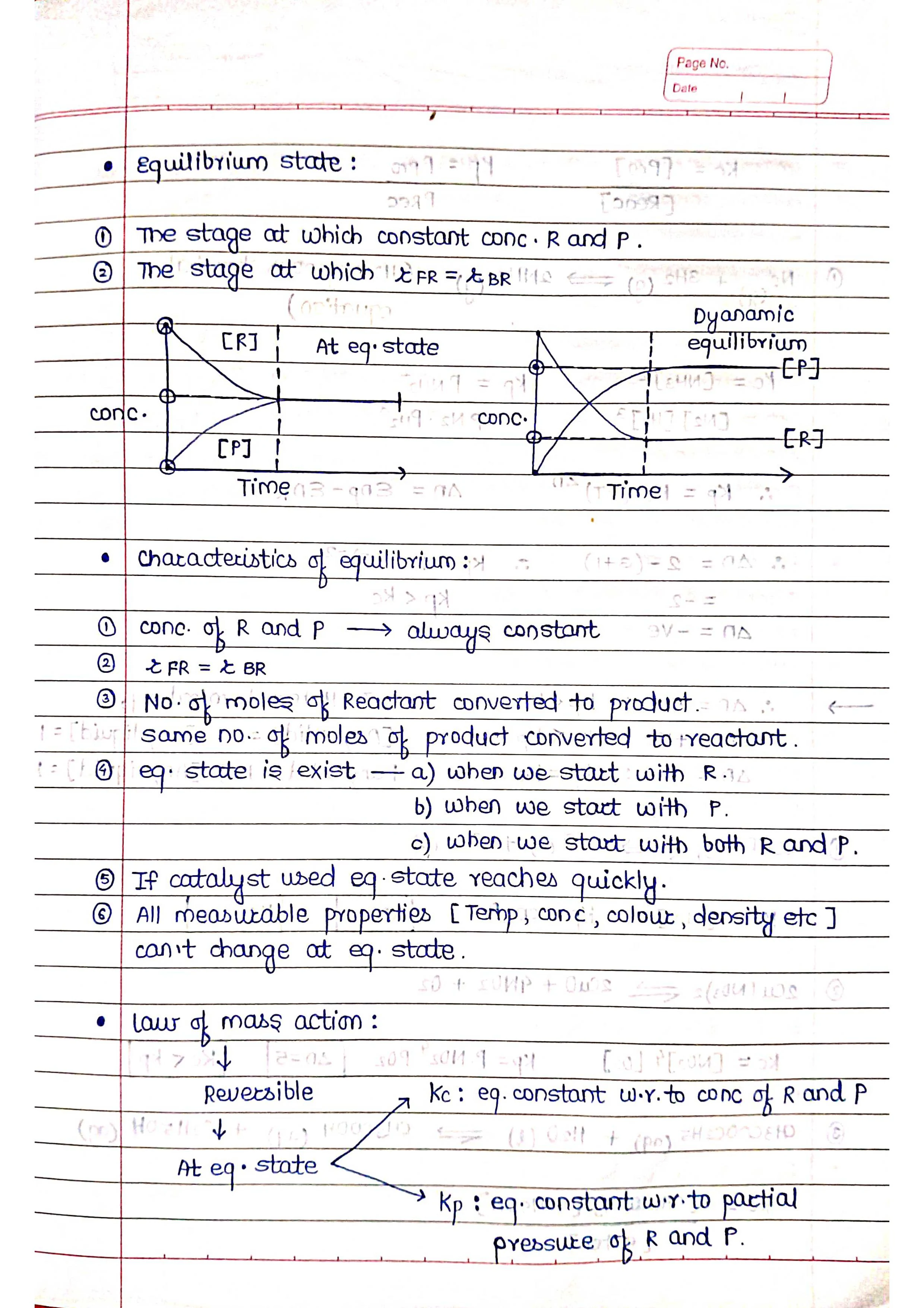
Chemical equilibrium is a state in which the rate of the forward reaction (reactants turning into products) is equal to the rate of the backward reaction (products turning back into reactants). This means that the concentrations of the reactants and products remain constant over time.
Key points about chemical equilibrium:
➭ It is achieved in reversible reactions, which are reactions that can go in both directions.
➭ At equilibrium, the system is dynamic, meaning that the forward and backward reactions are still happening, but at equal rates.
➭ The concentrations of the reactants and products at equilibrium are determined by the equilibrium constant (Kc), which is a specific value for each reaction at a given temperature.
➭ Factors that can affect the equilibrium position include changes in concentration, temperature, pressure, and the addition of a catalyst.
Here are some additional details about each of these points:
➭ Reversible reactions are often represented by a double arrow (⇌) to show that they can go in both directions. For example, the reaction between hydrogen and oxygen to form water is reversible:
2H₂ (g) + O₂ (g) ⇌ 2H₂O (g)
➭ The equilibrium constant (Kc) is a measure of the relative concentrations of the reactants and products at equilibrium. It is calculated by dividing the concentration of the products raised to their stoichiometric coefficients by the concentration of the reactants raised to their stoichiometric coefficients. For the reaction above, the equilibrium constant would be:
Kc = [H₂O]^2 / [H₂]^2 * [O₂]
➭ Changes in concentration can affect the equilibrium position. If the concentration of a reactant is increased, the forward reaction will be favored, and the equilibrium will shift to the right. Conversely, if the concentration of a product is increased, the backward reaction will be favored, and the equilibrium will shift to the left.
➭ Changes in temperature can also affect the equilibrium position. According to Le Chatelier's principle, exothermic reactions (reactions that release heat) are favored by lower temperatures, while endothermic reactions (reactions that absorb heat) are favored by higher temperatures.
➭ Changes in pressure can affect the equilibrium position for reactions that involve gases. If the pressure is increased on a system that contains gaseous reactants, the equilibrium will shift in the direction that produces fewer gas molecules (i.e., the direction that reduces the pressure).
➭ Catalysts can affect the rate of both the forward and backward reactions, but they do not affect the equilibrium position.
Chemistry Handwritten Short Notes 📚 for Class 11 & 12 | Free PDF Downloads
Chemistry Short Notes 📚⌛
1. Some Basic Concepts of Chemistry Short Notes 📚
2. Atomic Structure — Chemistry Short Notes 📚
3. Periodic table — Chemistry Short Notes 📚
4. Chemical Bonding — Chemistry Short Notes 📚
5. States of matter — Chemistry Short Notes 📚
6. Thermodynamics — Chemistry Short Notes 📚
7. P-Block Elements 1 - Chemistry Short Notes 📚
8. Ionic Equilibrium — Chemistry Short Notes 📚
9. Redox Reaction — Chemistry Short Notes 📚
10. Hydrogen — Chemistry Short Notes 📚
11. S-Block Elements - Chemistry Short Notes 📚



![Chemical Equilibrium - Chemistry Short Handwritten Notes [PDF]📚 Chemical Equilibrium - Chemistry Short Handwritten Notes [PDF]📚](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiDuQ7iULTyR9dG85tk3hxvYh5jGeFsxBkxwq6oaSS3IDauKQU-jTUWNVNJTXya2yhvmkpDaZ9fukUgcz_-wUsmi9m7IIzAZOuKPylpDr8AuXuKkSDg4nLRE1w-Pf5ItqG5al-wLODTdGPLLPBL5d10oXMLBULXVotvnc3v8pLBL0DEOwQ6CZ9v16MdI6s/s16000-rw/Chemical%20Equilibrium%20-%20Chemistry%20Short%20Handwritten%20Notes%20(2).jpeg)