The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structures and properties. Among the various blocks, the D and F blocks hold unique and fascinating characteristics. This article delves into these fascinating groups, exploring their electronic configurations, general properties, and diverse applications.
Introduction
➡️ The D block, also known as the transition metals, encompasses elements from Groups 3 to 12.
➡️ These elements possess partially filled d orbitals in their penultimate shell, leading to unique chemical behavior.
➡️ The F block, also known as inner transition metals, comprises two series: lanthanides (4f series) and actinides (5f series).
➡️ They are characterized by the filling of f orbitals in their outer shells.
D Block Elements: Unveiling Their Metallic Magic
➭ Electronic Configuration: D block elements exhibit partially filled (n-1)d orbitals, resulting in variable oxidation states and diverse bonding capabilities.
General Properties:
➭ Metallic: Excellent conductors of heat and electricity, malleable, and ductile.
➭ High Melting and Boiling Points: Strong metallic bonds contribute to their high thermal stability.
➭ Variable Oxidation States: Involvement of d electrons in bonding allows them to exhibit multiple oxidation states.
➭ Colorful Compounds: Partially filled d orbitals lead to the absorption of specific wavelengths of light, resulting in vibrant colors.
➭ Magnetic Properties: Unpaired electrons contribute to their paramagnetic or diamagnetic behavior.
➭ Alloy Formation: They readily form alloys with other metals, improving their mechanical properties.
➭ Catalytic Activity: Their ability to accelerate chemical reactions makes them valuable catalysts in various industrial processes.
➭ Examples: Iron (Fe), Copper (Cu), Silver (Ag), Gold (Au), Titanium (Ti), Nickel (Ni), Cobalt (Co).
F Block Elements: Delving into the Lanthanides and Actinides
Subcategories:
➭ Lanthanides (4f series): Elements from Ce to Lu.
➭ Actinides (5f series): Elements from Ac to Lr, with most being radioactive.
➭ Electronic Configuration: The filling of 4f orbitals (lanthanides) or 5f orbitals (actinides) defines their electronic structure.
General Properties:
➭ Metallic: Generally less reactive than d-block elements due to filled outer orbitals.
➭ Predominant +3 Oxidation State: Their stable electronic configurations lead to a preferred +3 oxidation state.
➭ Lanthanide Contraction: A gradual decrease in atomic and ionic radii across the lanthanide series due to increased nuclear charge.
➭ Radioactivity in Actinides: Most actinides are radioactive due to unstable nuclei, making them valuable in nuclear energy but posing environmental concerns.
➭ Examples: Lanthanum (La), Cerium (Ce), Gadolinium (Gd), Ytterbium (Yb), Plutonium (Pu), Uranium (U).
D-Block Elements
➡️ The d-block elements are present from the fourth period onwards in the periodic table.
➡️ They are mainly divided into three series: 3d series (Sc to Zn), 4d series (Y to Cd), and 5d series (La to Hg, omitting Ce to Lu).
➡️ D-block elements are also known as transition elements because of their position between the s-block and p-block elements in the periodic table.
➡️ Their electronic configuration is (n – 1)d1-10ns2. However, Cu+, Zn, Cd, Hg etc. [(n – 1)d10] are exceptions as they have completely filled d-orbitals and are not considered true transition metals.
General Properties of the Transition Elements
➭ Atomic and Ionic Radii: Due to poor shielding effect, the atomic and ionic radii of transition elements decrease from left to right for the first five elements in a series, and then become almost constant for the next five elements.
➭ Enthalpies of Atomization: Transition elements have higher enthalpies of atomization due to the large number of unpaired electrons in their atoms, resulting in stronger interatomic interactions.
➭ Ionization Enthalpies: The ionization enthalpies generally increase from left to right in a series due to the increasing nuclear charge.
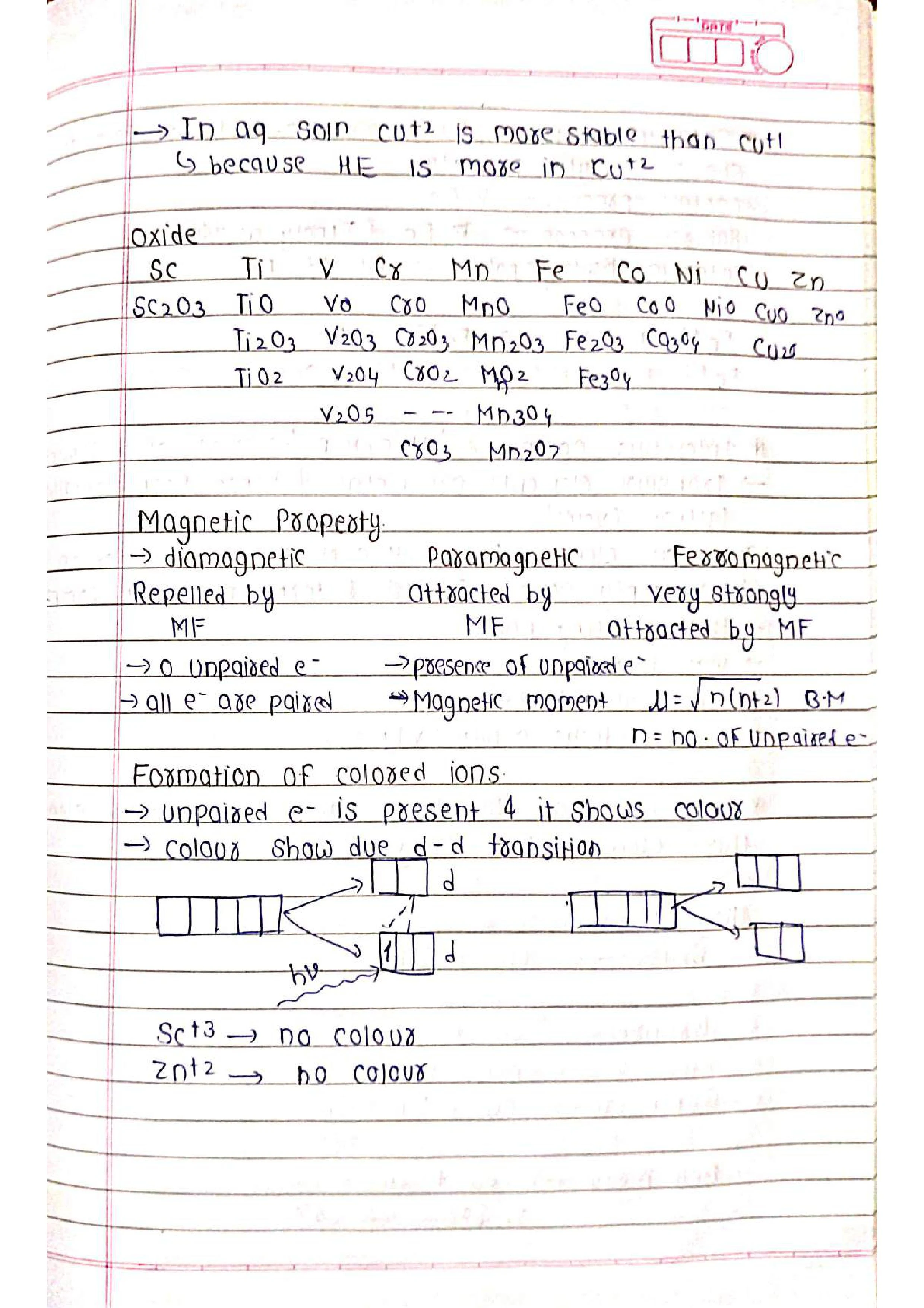
➭ Oxidation States: Transition elements exhibit variable oxidation states due to the presence of incomplete outermost shells. The stable oxidation states of the first-row transition metals are Sc(+3), Ti(+4), V(+5), Cr(+3, +6), Mn(+2, +7), Fe(+2, +3), Co(+2, +3), Ni(+2), Cu(+2), Zn(+2).
➭ Trends in Standard Electrode Potentials: The standard electrode potentials of transition metals depend on the sum of the first and second ionization enthalpies. If this sum is greater than the hydration enthalpy, the standard electrode potential will be positive, and the reactivity will be lower.
➭ Magnetic Properties: Transition elements exhibit paramagnetism due to the presence of unpaired electrons. The magnetic moment is determined by the number of unpaired electrons according to the formula n = √(n(n + 2)), where n is the number of unpaired electrons.
➭ Formation of Coloured Ions: The presence of d-orbitals allows for the absorption of specific wavelengths of light, resulting in the observed colors of transition metal ions.
➭ Formation of Complex Compounds: Transition metals can form complex compounds due to their small size, high nuclear charge, and vacant d-orbitals.
➭ Catalytic Properties: Transition metals can act as catalysts due to their multiple oxidation states and ability to form complexes.
➭ Formation of Interstitial Compounds: Small non-metals like H, C, and N can fit into the voids of transition metals, forming interstitial compounds with high melting points, hardness, and metallic conductivity.
➭ Alloy Formation: Transition metals can form homogeneous mixtures called alloys with other metals due to their similar sizes.
Some Important Compounds of Transition Elements
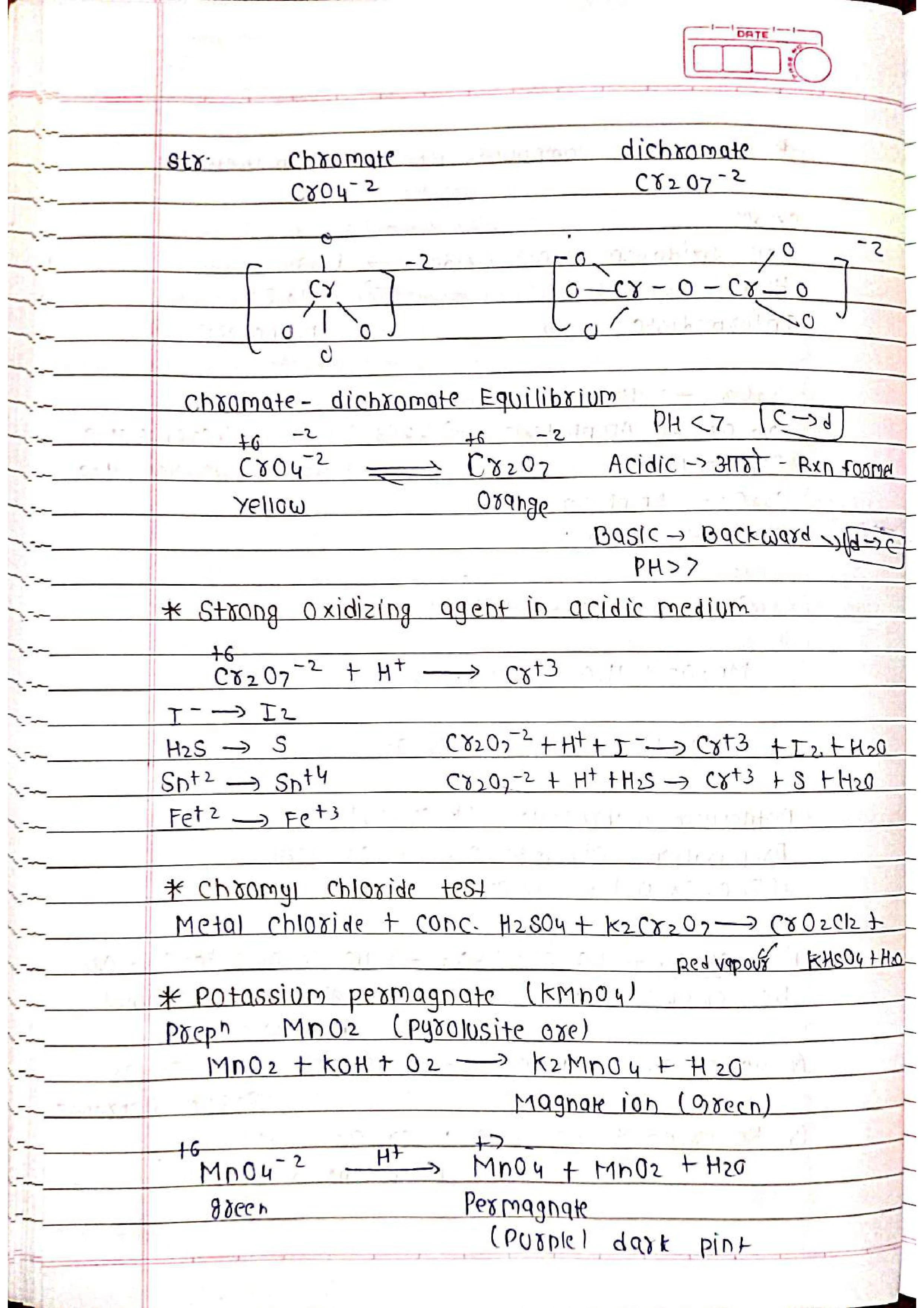
➭ Potassium Dichromate (K2Cr2O7):
Used as an oxidizing agent in various applications, including volumetric analysis, leather tanning, and photography.
➭ Potassium Permanganate (KMnO4):
A strong oxidizing agent used in disinfection, water purification, and organic synthesis.
Inner Transition Elements (f-Block)
➭ The f-block elements consist of two series: lanthanoids and actinoids.
➭ Lanthanoids are known as rare earth metals, while actinoids are radioactive elements.
Lanthanoids
General characteristics include:
➭ Electronic configuration [Xe] 4f1-14, 5d0-1, 6s2.
➭ Decrease in atomic and ionic size from left to right due to lanthanoid contraction.
➭ Silvery white, soft metals that tarnish easily in air.
➭ Many trivalent lanthanoid ions are colored.
➭ Paramagnetic due to unpaired f-electrons.
➭ Exhibit variable oxidation states, with +3 being the most common.
➭ Misch metal, an alloy of lanthanoids, is used in cigarette ligh