Chemical bonding is the force that holds atoms together to form molecules or crystals. It's the reason why table salt (NaCl) is a solid crystal, and why water (H₂O) is a liquid. Bonding is all about stability: atoms want to achieve a stable electron configuration, and they can do that by bonding with other atoms.
There are three main types of chemical bonds:
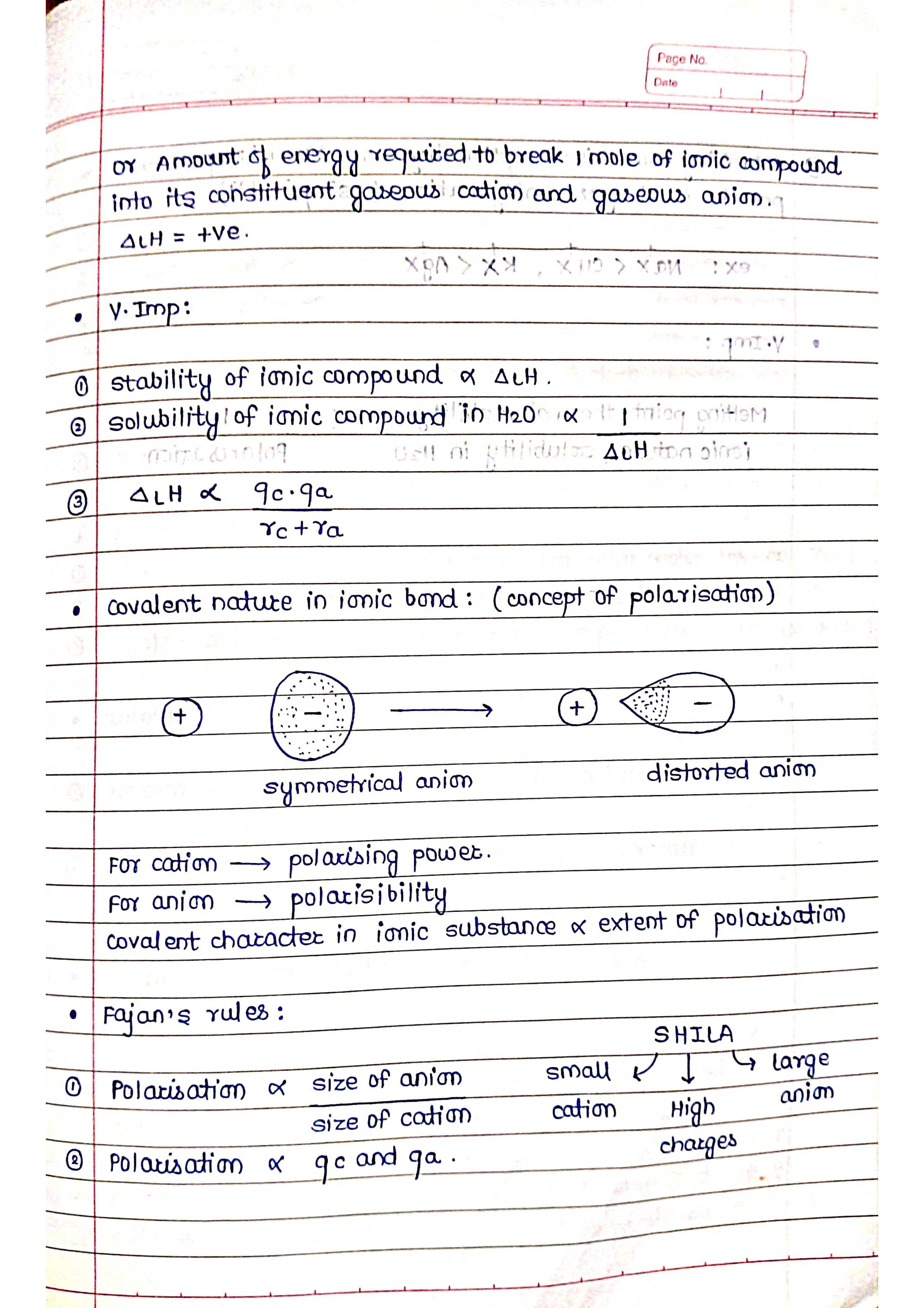
Ionic bonding: This type of bond occurs when one atom transfers an electron to another atom. The atom that loses the electron becomes positively charged (cation), and the atom that gains the electron becomes negatively charged (anion). The oppositely charged ions attract each other, forming an ionic bond.
Covalent bonding: This type of bond occurs when two atoms share electrons. Each atom contributes one or more electrons to the shared pool, and the attraction between the electrons and the nuclei of both atoms holds the atoms together. Covalent bonds can be single, double, or triple, depending on the number of electrons shared.
Metallic bonding: This type of bond occurs in metals. In metals, the valence electrons are delocalized, meaning they are not bound to any particular atom. These delocalized electrons form a "sea" of electrons that surrounds the positively charged metal ions. The attraction between the delocalized electrons and the metal ions holds the metal together.
The type of bond that forms between two atoms depends on their electronegativities. Electronegativity is a measure of an atom's tendency to attract electrons towards itself. The greater the difference in electronegativity between two atoms, the more likely they are to form an ionic bond. If the atoms have similar electronegativities, they are more likely to form a covalent bond.
Chemical bonding is a fundamental concept in chemistry, and it is essential for understanding the properties of matter. By understanding the different types of bonds and the factors that affect them, we can predict how atoms will interact with each other and form new compounds.
Chemistry Handwritten Short Notes 📚 for Class 11 & 12 | Free PDF Downloads
Here are some additional points to remember about chemical bonding:
- Bonds can be strong or weak. Strong bonds require more energy to break than weak bonds.
- Bonds can be polar or nonpolar. A polar bond is a covalent bond in which the electrons are shared unequally between the two atoms. A nonpolar bond is a covalent bond in which the electrons are shared equally between the two atoms.
- The geometry of a molecule is determined by the arrangement of its bonds. The shape of a molecule can affect its properties.
Chemistry Short Notes 📚⌛
1. Some Basic Concepts of Chemistry Short Notes 📚
2. Atomic Structure — Chemistry Short Notes 📚
3. Periodic table — Chemistry Short Notes 📚
4. P-Block Elements 1 - Chemistry Short Notes 📚
5. States of matter — Chemistry Short Notes 📚
6. Thermodynamics — Chemistry Short Notes 📚
7. Chemical Equilibrium — Chemistry Short Notes 📚
8. Ionic Equilibrium — Chemistry Short Notes 📚
9. Redox Reaction — Chemistry Short Notes 📚
10. Hydrogen — Chemistry Short Notes 📚
11. S-Block Elements - Chemistry Short Notes 📚