Thermal Properties of Matter - Physics delves into the fundamental nature of our universe, and the behavior of matter under various conditions is a crucial area of study. Thermal properties are a fascinating aspect of physics, encompassing how different materials respond to changes in temperature. This knowledge is essential in various fields, from designing buildings to engineering spacecraft.
What are Thermal Properties of Matter?
Thermal properties are inherent characteristics of matter that govern its interaction with heat. These properties determine how a material absorbs, transfers, and releases thermal energy. In essence, they dictate how a substance behaves when heated or cooled.
Types of Thermal Properties
There are several key thermal properties that influence a material's response to temperature changes:
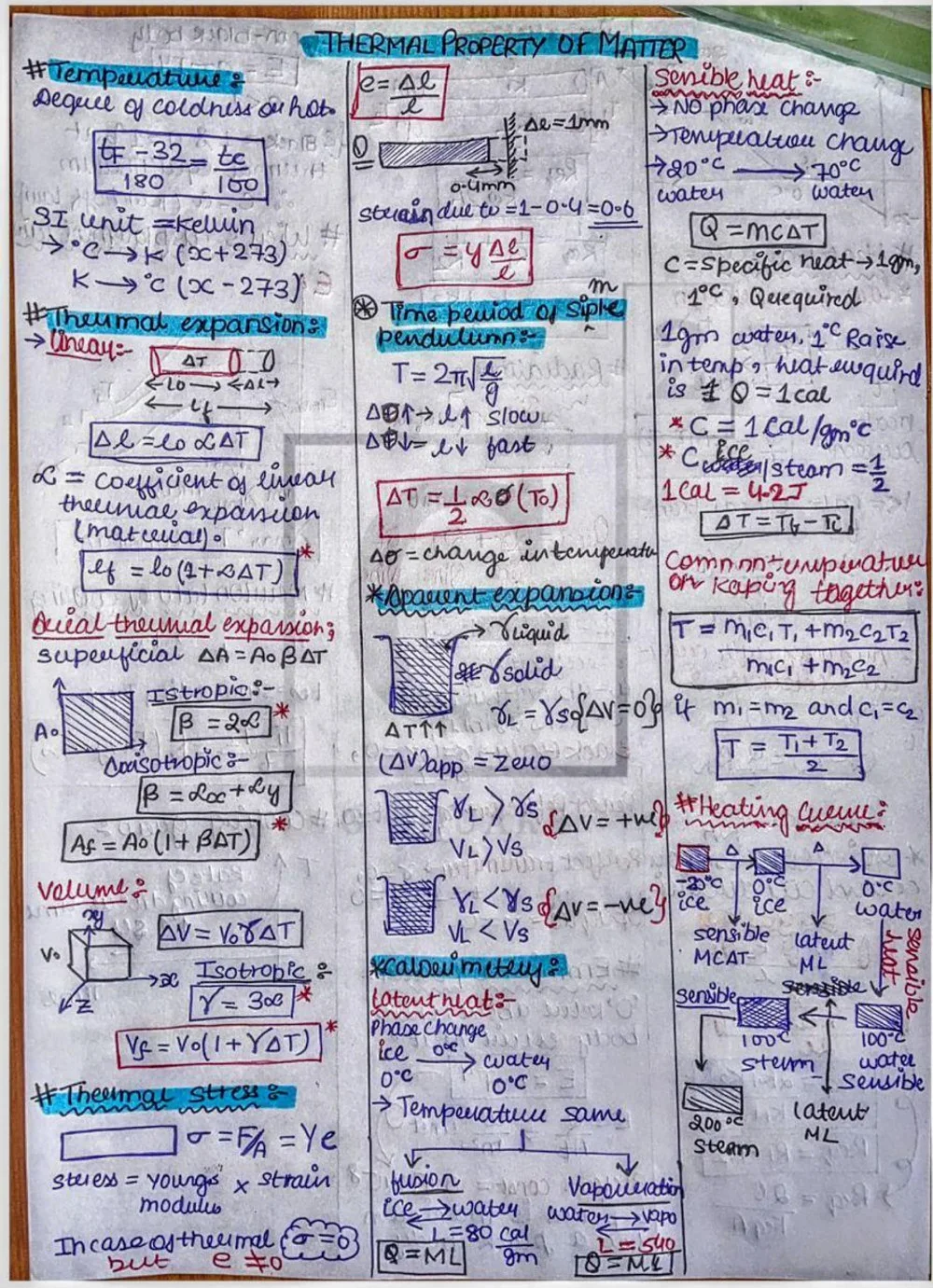
⦿ Specific Heat Capacity: This property signifies the amount of heat energy required to raise the temperature of a unit mass of a substance by one unit of temperature. Materials with high specific heat capacities require more heat to experience a temperature increase, making them good for thermal storage applications. Water, for instance, has a high specific heat capacity.
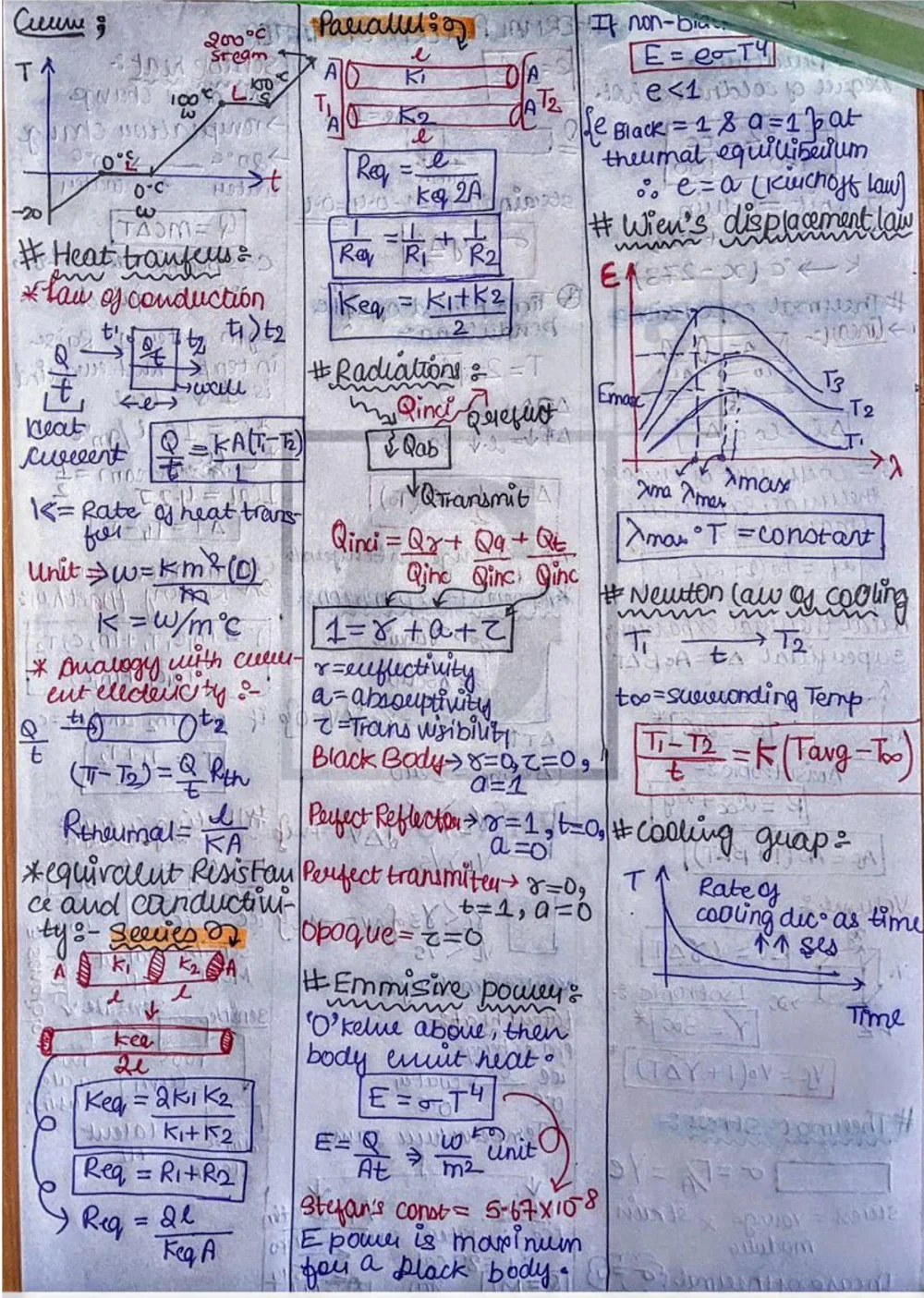
⦿ Thermal Conductivity: This property quantifies a material's ability to conduct heat. Materials with high thermal conductivity readily transfer heat from hotter regions to cooler regions. Metals like copper and aluminum are excellent thermal conductors, while materials like wood or plastic are poor conductors, making them suitable for thermal insulation.
⦿ Thermal Expansion: This property describes the tendency of a material to change its size (volume, area, or length) in response to temperature variations. Most materials expand upon heating and contract upon cooling. This property is crucial for engineering applications, as it needs to be considered to prevent stress or damage in structures due to temperature fluctuations.
⦿ Melting Point and Boiling Point: These properties represent the specific temperatures at which a solid transitions to a liquid (melting) and a liquid transforms into a gas (boiling). These phase changes are driven by the thermal energy supplied to the material.
Examples of Thermal Properties in Action
⦿ Cooking Utensils: Pans and pots made of metals like aluminum or copper are preferred for cooking due to their high thermal conductivity, ensuring even heat distribution for efficient cooking.
⦿ Building Insulation: Materials like fiberglass or rockwool are used as building insulation because of their low thermal conductivity, minimizing heat transfer and maintaining comfortable indoor temperatures.
⦿ Thermometers: These instruments utilize the principle of thermal expansion, where a specific liquid (like mercury) expands with increasing temperature, allowing for temperature measurement.
Formula for Thermal Properties
⦿ Specific Heat Capacity (c): Q = mcΔT (where Q is heat energy transferred, m is mass, c is specific heat capacity, and ΔT is the change in temperature)
⦿ Thermal Conductivity (k): q = -kAΔT/Δx (where q is the heat flux, k is thermal conductivity, A is the cross-sectional area, ΔT is the temperature difference, and Δx is the distance)
⦿ Thermal Expansion (α): ΔL = L₀αΔT (where ΔL is the change in length, L₀ is the original length, α is the coefficient of thermal expansion, and ΔT is the change in temperature)
Specific Heat Capacity (c): c = Q / (m ΔT)
Q: Heat energy transferred (J)
m: Mass of the substance (g)
ΔT: Change in temperature (°C or K)
Thermal Conductivity (k): Q = -kA (ΔT / Δx)
Q: Heat energy transferred (J)
k: Thermal conductivity of the material (W/m⋅K)
A: Cross-sectional area (m²)
ΔT: Temperature difference (°C or K)
Δx: Distance (m)
By understanding these thermal properties and the relevant formulas, we can effectively predict and manipulate the behavior of materials under varying temperature conditions. This knowledge is instrumental in numerous fields, from engineering and material science to thermodynamics and climate control.
FAQs
Why does metal feel colder than wood at the same temperature?
This perception arises because of a concept called thermal diffusivity. While both metal and wood may be at the same temperature, metal has a higher thermal conductivity. When you touch metal, heat from your body transfers to the metal at a faster rate, making the metal feel colder. Wood, with lower thermal conductivity, conducts heat away from your body slower, resulting in a less pronounced cooling sensation.
Can thermal properties change over time?
In some cases, yes. Certain materials can undergo physical or chemical changes that can affect their thermal properties. For instance, repeated heating and cooling cycles can degrade some polymers, reducing their thermal conductivity. Additionally, some materials exhibit changes in thermal properties depending on their state (solid, liquid, gas).
What are negative thermal expansion coefficients?
While most materials expand upon heating, a rare few exhibit the opposite behavior. Materials with negative thermal expansion coefficients actually contract when heated and expand when cooled. Examples include water at temperatures below 4°C and some specific alloys.
How are thermal properties used in nanotechnology?
Nanotechnology deals with materials at the atomic and molecular level. Here, thermal properties play a crucial role. Scientists can engineer materials with specific thermal properties by manipulating their structure at the nanoscale. This allows for the development of materials with exceptional heat dissipation capabilities or highly targeted thermal responses for various applications.
What are some everyday applications of thermal properties besides the ones mentioned?
Thermal properties find applications in various aspects of our daily lives. Here are a few examples:
⦿ Spoons in hot beverages: Metal spoons conduct heat away from the hot beverage, making them easier to hold.
⦿ Bimetallic strips: These strips, composed of two metals with different thermal expansion coefficients, are used in thermostats because they bend with temperature changes, triggering on/off mechanisms.
⦿ Self-defrosting freezers: The evaporator coils in these freezers utilize the principle of thermal conductivity to efficiently remove frost buildup.